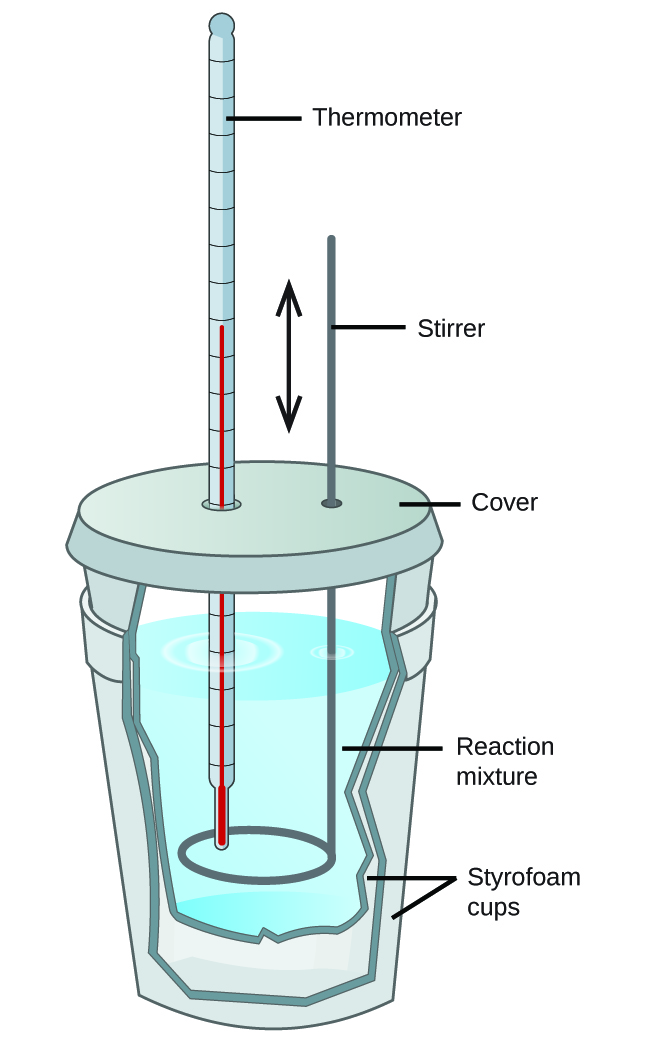
Calorimetry Simple Definition . Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical.a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
from rainis.pics
calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity.a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.
Calorimeter Types and Heat Flow Analysis (M6Q5) UWMadison Chemistry
Calorimetry Simple Definition a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical.calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for.
From glossary.periodni.com
Bomb calorimeter Chemistry Dictionary & Glossary Calorimetry Simple Definitioncalorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for.a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. calorimetry is the measurement of the transfer of heat into or out of a system. Calorimetry Simple Definition.
From www.thoughtco.com
Calorimeter Definition in Chemistry Calorimetry Simple Definitiona calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical.calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.. Calorimetry Simple Definition.
From www.youtube.com
050 Calorimetry YouTube Calorimetry Simple Definitioncalorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical.a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.a calorimeter is. Calorimetry Simple Definition.
From haipernews.com
How To Calculate Heat Capacity Of Air Haiper Calorimetry Simple Definitioncalorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical.a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical. Calorimetry Simple Definition.
From serc.carleton.edu
Calorimetry Calorimetry Simple Definitioncalorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical.a calorimeter is a device used. Calorimetry Simple Definition.
From igcse-chemistry-2017.blogspot.com
IGCSE Chemistry 2017 3.2 Describe Simple Calorimetry Experiments for Calorimetry Simple Definition calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical.calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for.a calorimeter. Calorimetry Simple Definition.
From study.com
Calorimetry Definition, Equation & Types Lesson Calorimetry Simple Definition calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical.calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for.a calorimeter. Calorimetry Simple Definition.
From pressbooks.online.ucf.edu
10.2 Calorimetry Chemistry Fundamentals Calorimetry Simple Definitioncalorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. Calorimetry is a field of thermochemistry that measures the amount of heat involved in a. Calorimetry Simple Definition.
From www.youtube.com
CHEMISTRY 101 Constant volume calorimetry YouTube Calorimetry Simple Definitiona calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity.calorimetry is the process of measuring the amount of heat released or. Calorimetry Simple Definition.
From people.chem.umass.edu
Untitled Document [people.chem.umass.edu] Calorimetry Simple Definitioncalorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity.calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction. Calorimetry Simple Definition.
From grade12uchemistry.weebly.com
Calorimetry Grade12UChemistry Calorimetry Simple Definitiona calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical.a calorimeter is a device used to measure the amount. Calorimetry Simple Definition.
From atanchem.weebly.com
AP Chem Chemistry LHS Calorimetry Simple Definitiona calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity.a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.calorimeter, device for measuring the heat developed during a mechanical, electrical, or. Calorimetry Simple Definition.
From www.youtube.com
Principle of Calorimetry YouTube Calorimetry Simple Definition Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical.a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes. Calorimetry Simple Definition.
From sagote.com
Máy đo năng lượng, Calori Meter Sagote, Mr Bắc 0986655594 Calorimetry Simple Definitiona calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity.a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. Calorimetry is a field of thermochemistry that measures the amount of heat involved. Calorimetry Simple Definition.
From www.youtube.com
Thermal Properties of Matter Class 11 Physics Calorimetry Principle Calorimetry Simple Definitioncalorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.a calorimeter is a device used for calorimetry, or the process of measuring the heat of chemical reactions or physical changes as well as heat capacity. calorimetry is the measurement of the transfer of heat into or out of a. Calorimetry Simple Definition.
From www.pathwaystochemistry.com
Calorimetry Pathways to Chemistry Calorimetry Simple Definition Calorimetry is a field of thermochemistry that measures the amount of heat involved in a physical or chemical. calorimetry is the measurement of the transfer of heat into or out of a system during a chemical reaction or physical.calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.a. Calorimetry Simple Definition.
From rainis.pics
Calorimeter Types and Heat Flow Analysis (M6Q5) UWMadison Chemistry Calorimetry Simple Definitiona calorimeter is a device used to measure the amount of heat involved in a chemical or physical process.calorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for.calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction. Calorimetry is a field of. Calorimetry Simple Definition.
From www.tessshebaylo.com
Equation For Determining Calorimetry Tessshebaylo Calorimetry Simple Definitioncalorimeter, device for measuring the heat developed during a mechanical, electrical, or chemical reaction and for.calorimetry is the process of measuring the amount of heat released or absorbed during a chemical reaction.a calorimeter is a device used to measure the amount of heat involved in a chemical or physical process. calorimetry is the measurement. Calorimetry Simple Definition.